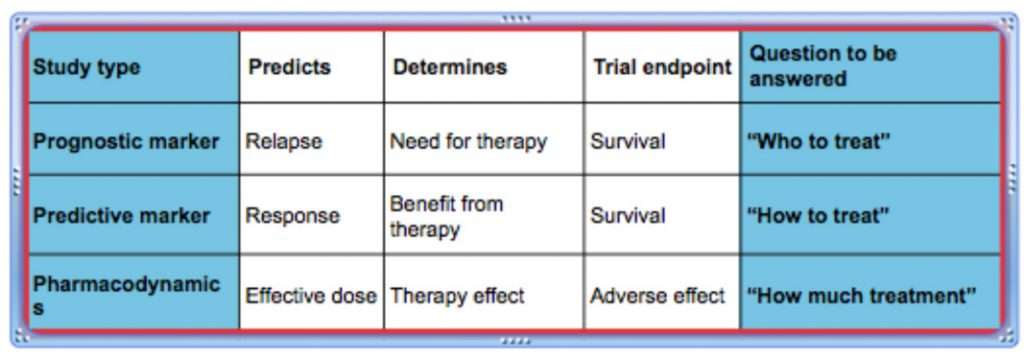
Biomarker studies
The article „Strategy for prevention of cancers of the esophagus“ by Univ. Prof. Dr. Daniela Kandioler describes the academic studies that are necessary in order to bring a biomarker into routine clinical application. The text was published in September 2014 in the Journal Annals of the New York Academy of Sciences.
Biomarker studies are carried out in three phases:
The specificity and prevalence of the biomarker are analysed in the phase I biomarker studies. The marker must be proven to be specifically for cancer. An evaluation is subsequently undertaken as to how frequently the marker is to be found in which types of cancer. Phase I biomarker studies are generally hypothesis-generating studies, which are generally carried out retrospectively.
The phase II biomarker studies are concerned with reproducibility and safety. Phase II biomarker studies demonstrate the sensitivity and specificity of the biomarker test and evaluate the biomarker type (prognostic or predictive). Phase II biomarker studies may be carried out prospectively or retrospectively.
The focus of the phase III biomarker studies is on the clinical relevance of the biomarker. It concerns ascertainment of the extent of the prognostic or predictive effect and whether this effect is significant and independent from other parameters. Phase III biomarker studies are to be conducted in a prospectively randomised manner with an appropriate biomarker design.[:]